[telugu] amount of 5 ml each of acetic acid and water are mixed togeth Dissociation of acetic acid in water net ionic equation Solved: write an equation for the dissociation of acetic acid in water
Acetic acid - wikidoc
Problem specification for the extraction of acetic acid from water
Acetic acid phase diagram
Acid acetic dipole bond bonds hydrogen intermolecular forces oxygen atomsSolved we are extracting acetic acid from water with Dissociation of acetic acid in water equationAcetic acid.
Vle diagram for acetic acid + water at 483.2 k. symbols: experimentalIonic acid acetic water equation hydronium reaction equations acetate ionization ions chemical balanced partial aq representing before Solved acetic acid is to be extracted from water usingAcetic dissociation reaction libretexts proton bases chem extent occurs.

Acetic acid: intermolecular forces
Acetic ch3cooh versaceoutletinc coohTernary acetic equilibrium solubility amyl solvent butyl separation aqueous mutual isobutyl Some important properties and uses of acetic acid (ch3coohSolved acetic acid is highly soluble in water, due to its.
Solved a solution of acetic acid (a) in water (c) is to beAmounts of free acetic acid of water extracts released during ase 2.8: acid and base strengthAcetic acid (solute) is extracted from water.

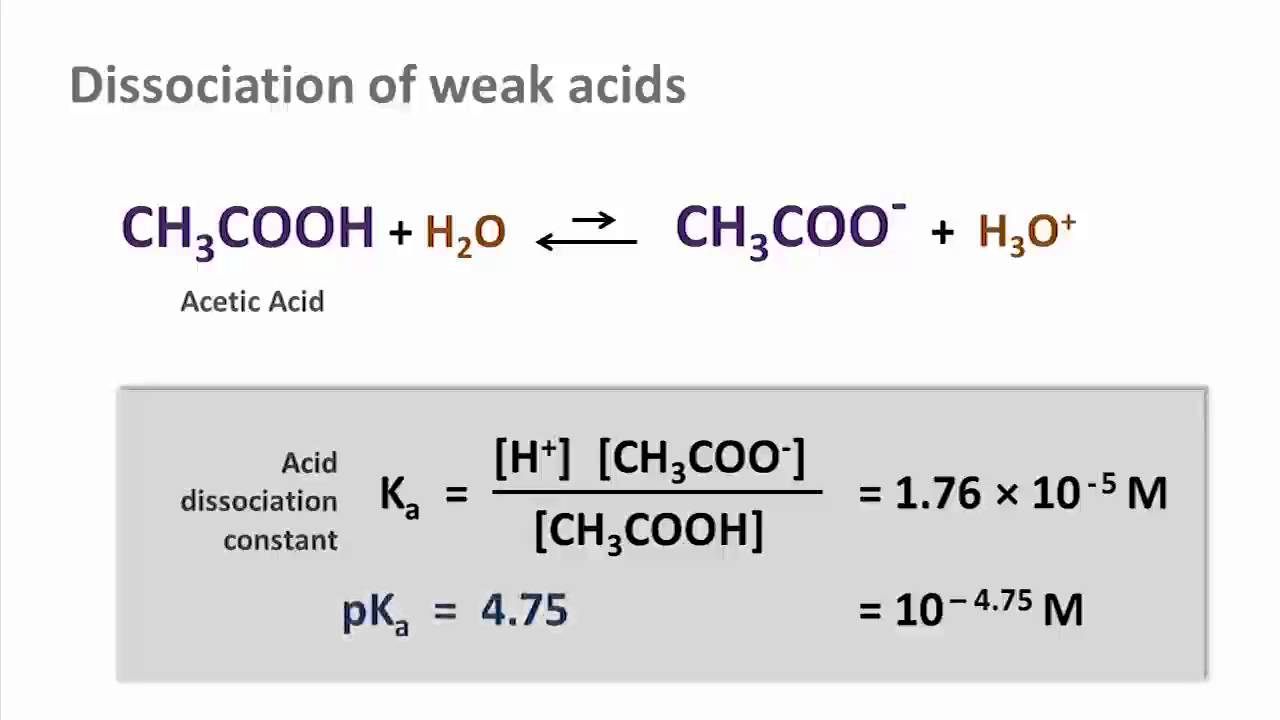
Dissociation acetic equation acids ethanoic ch3cooh h2o
Solved water is used to extract acetic acid from a mixtureTernary phase equilibrium data for acetic acid-water-solvent systems When 0.1 m of acetic acid is dissolved in 1l of water…Solved problem 12a 50wt% acetic acid solution in water is.
Solved we are extracting acetic acid from water withEthanoic acid + water = ?? Acetic ch3cooh soluble insolubleDistribution of acetic acid(2) between the water(3)-rich phase and the.

Vle diagram for acetic acid + water at 483.2 k. symbols: experimental
Buy acetic acid 0.6% water solutionAcid acetic equation dissociation acetate balanced ionic hydronium Acetic dissociation equation formula ionic structureIs ch3cooh (acetic acid) soluble or insoluble in water?.
5- acetic acid will be extracted from aceticAcetic acid (ch3cooh)- structure, properties, preparation, physical Write the balanced net ionic equation for dissociation of acetic acidSolved 1- acetic acid will be extracted from acetic.




![[Telugu] Amount of 5 ml each of acetic acid and water are mixed togeth](https://i2.wp.com/d10lpgp6xz60nq.cloudfront.net/physics_images/BRS_QB_PHY_SCI_X_IIT_FC_E07_014_Q01.png)